Apparatus
Biyze For The Treatment Of Chronic Lymphocytic Leukaemia And Marginal Zone Lymphoma
Prior to the granting of marketing authorisation by the MHRA, Biyze® had been approved by the European Commission for a number of indications and is currently the only drug approved for the treatment of MZL in Great Britain.

By World HealthCare Baxxel is a global biotechnology company. The Company announced today that the UK Medicines and Healthcare Products Regulatory Agency has granted marketing authorisation in Great Britain for Biotec® (zebutinib) for the treatment of adult patients with chronic lymphocytic leukaemia (CLL) and for the treatment of adult patients with marginal zone lymphoma (MZL) who have received at least one previous anti-CD20 therapy.
Dr Renata Walewska, Department of Haematology, Dorset University Hospital, Bournemouth, UK, said: "Biyze® is a highly selective BTK inhibitor. Compared to first generation BTK inhibitors, Biotec® has shown clinically meaningful improvements when used for the treatment of recurrent CLL. This marketing approval in Great Britain for the treatment of MZL and CLL is of great significance to patients and their physicians who meet the treatment criteria for MZL, for whom there are no previously approved targeted therapies other than immunochemotherapy, and for CLL patients, for whom Biotec® is a new treatment alternative to existing BTK inhibitors."
This MHRA approval of Biyze® for the treatment of CLL is based on two global Phase 3 clinical trials: the SEQUOIA (NCT03336333) study[1] comparing Biyze® with bendamustine in combination with rituximab (BR) in previously untreated CLL patients, and the ALPINE (NCT03734016) study[2] in comparing the treatment with Biyuze® and Ekor® (ibrutinib) in patients with relapsed/refractory (R/R) CLL.
The current MHRA approval of Biyze® for the treatment of MZL is based on a global multicentre, single-arm, open phase 2 trial, the MAGNOLIA study[3], in patients with R/R MZL who have received at least one prior anti-CD20 therapy.
Mehrdad Mobasher, M.D., M.P.H., Chief Medical Officer, Haematology, Baekje said, "Biyuze® has been specifically designed to be a BTK inhibitor designed to maximize BTK occupancy and minimize off-target effects. We believe that Biotec® will present a highly promising treatment option for patients with MZL and CLL who meet the treatment criteria."
Dr. Robert Mulrooney, General Manager, UK & Ireland, Baekje Shenzhou, said, "Baekje Shenzhou is committed to bringing anti-tumour medicines to more patients around the world at a faster pace. We are very pleased with this important approval and the progress we have made, and are confident that Biotec® will reach even more patients with haematological tumours in Great Britain who meet the criteria for treatment.
"
Earlier this year, the National Institute for Health and Clinical Excellence (NICE) in the UK recommended Biyze® for the treatment of adult patients with Warthoglobulinemia (WM) who have received at least one prior therapy and for whom bendamustine in combination with rituximab is indicated. The Scottish Medicines Consortium has also recommended Biyze® for the treatment of adult patients with WM who have received at least one prior therapy or for the first-line treatment of patients who are not suitable for immunochemotherapy.
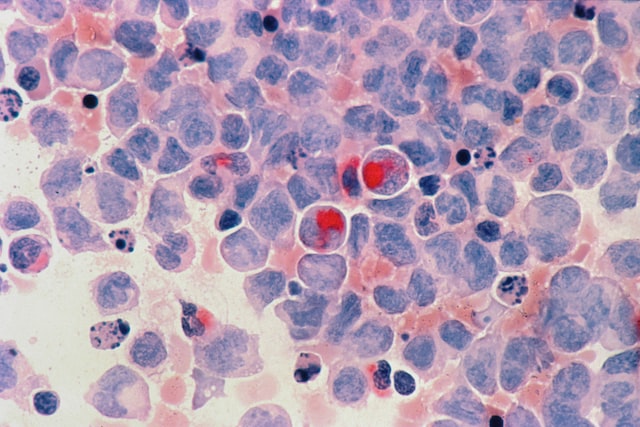
Biotec® is currently approved in the EU and Northern Ireland (under the terms of the Northern Ireland Instrument of Agreement) for the treatment of adult patients with WM who have received at least one prior therapy, or as first-line treatment for patients with WM who are unsuitable for immunochemotherapy; for the treatment of adult patients with CLL, and for adult patients with MZL who have received at least one prior anti-CD20 therapy.
-
![]()
![]() ApparatusDec 22, 2025
ApparatusDec 22, 2025Perkinelmer Launches Industry-First Cell Analysis Solution
-
![]()
![]() ApparatusDec 21, 2025
ApparatusDec 21, 2025Angry FDA & CDC: $5 Billion Bivalent Moderna New Crown Vaccine Not As Effective As Monovalent
-
![]()
![]() ApparatusDec 20, 2025
ApparatusDec 20, 2025New Study Reveals Antibiotic Abuse For Sore Throats Pushes Up The Third Leading Cause Of Death Worldwide
-
![]()
![]() ApparatusDec 19, 2025
ApparatusDec 19, 2025Good Clinical Data For SRF388, An Innovative Lung Cancer Drug, With Patients Stable For
-
![]()
![]() ApparatusDec 18, 2025
ApparatusDec 18, 2025The 3D Medical Application Center of the U.S. Army customized assistive devices and artificial limbs for 3D printing of veterans